Opals are one of the most fascinating gemstones in the world, known for their mesmerizing play of colors. While opals come in various shades and hues, black and white opals are particularly sought after for their unique beauty. Have you ever wondered what gives black opal its dark color and white opal its light color? And why are some opals crystal and transparent? Let's dive into the chemical composition of these captivating gemstones to find out.
What Makes Black Opal Black?
Black opals are prized for their deep, dark body color, which ranges from black to dark gray. The secret behind their captivating darkness lies in their chemical composition. Black opals contain a higher percentage of a mineral called carbon compared to other opals. This carbon content absorbs more light, resulting in a darker appearance. The presence of iron oxide and manganese oxide also contributes to the black opal's coloration.
What is Black Opal?
Black opal is a type of opal that displays a dark body color, ranging from deep blue to black. Unlike other opals, black opal is characterized by its vibrant play of colors, which can include hues of red, green, blue, and purple. This captivating phenomenon is known as opalescence.
The Chemical Makeup of Black Opal
Black opal is primarily composed of hydrated silica, also known as silicon dioxide (SiO2). However, what sets black opal apart from other opals is the presence of microscopic spheres of silica within its structure. These spheres diffract light, resulting in the play of colors that black opal is renowned for.
In addition to silica, black opal may contain trace elements such as iron, manganese, and carbon. These impurities can influence the color and intensity of the opalescence, giving each black opal its unique character.
The Role of Water in Black Opal
Water plays a crucial role in the formation of black opal. During the process of opalization, silica-rich water seeps into cracks and cavities in the earth. Over time, this water evaporates, leaving behind deposits of silica. The arrangement of these silica deposits, along with the presence of water, contributes to the formation of the opal's unique structure.
The water content in black opal can vary, ranging from a few percent to as high as 20%. The amount of water present affects the opal's stability and durability. Opals with higher water content are generally more prone to cracking and dehydration.
The Geological Origins of Black Opal
Black opal is primarily found in Australia, particularly in the Lightning Ridge region of New South Wales. The geological conditions in this area, including the presence of ancient sedimentary rocks and volcanic activity, have created the perfect environment for the formation of opals.
During the Cretaceous period, around 100 million years ago, silica-rich gel formed in the sedimentary rocks. Over time, this gel solidified and transformed into opal. The unique combination of geological processes, including the presence of ironstone and the absence of oxygen, contributed to the formation of black opal.
The chemical composition of black opal, with its hydrated silica and microscopic spheres, is what gives this gemstone its captivating play of colors. The presence of trace elements and water content further enhance its beauty. Understanding the scientific details behind black opal allows us to appreciate its rarity and uniqueness. So, the next time you admire a black opal, remember the intricate chemistry that lies within.
Why is White Opal White?
White opals, on the other hand, exhibit a lighter body color, ranging from milky white to light gray. The primary reason behind their paler appearance is the absence of carbon in their chemical composition. Without the presence of carbon, white opals do not absorb as much light, resulting in a lighter color. The white opal's body color is often complemented by vibrant flashes of color, known as play-of-color, which is a hallmark of opals.
What is White Opal?
White opal, also known as milky opal, is a type of precious opal that displays a white or light-colored body tone. It is characterized by its play-of-color, which refers to the vibrant flashes of rainbow colors that dance across the surface of the gemstone when it is viewed from different angles. These captivating colors are what make white opal so highly prized by gem enthusiasts and collectors.
The Chemical Formula
The chemical composition of white opal is primarily composed of hydrated silica, also known as silicon dioxide (SiO2), with a water content ranging from 3% to 21%. The presence of water molecules within the silica structure is what gives opal its unique optical properties. The water content in white opal is responsible for diffracting light, resulting in the play-of-color phenomenon.
Microstructure and Formation
White opal is formed through a process called opalization, which occurs when silica-rich water seeps into cracks and voids in rocks, such as sandstone or basalt. Over time, the water evaporates, leaving behind a gel-like substance that solidifies into opal. The microstructure of white opal consists of tiny spheres of silica arranged in a three-dimensional lattice. These spheres diffract light, causing the play-of-color effect.
Trace Elements and Impurities
While the main component of white opal is silica, it can also contain trace elements and impurities that influence its appearance. Common impurities found in white opal include iron oxide, which can give the gemstone a yellowish or reddish tint, and carbon, which can create a grayish undertone. These impurities, along with the arrangement of the silica spheres, contribute to the overall color and character of white opal.
The chemical composition of white opal consists of hydrated silica, with a water content that gives it its unique optical properties. The microstructure, trace elements, and impurities all play a role in the appearance of white opal. Understanding the chemical composition of this captivating gemstone adds to the appreciation of its natural beauty. Whether you're a gem enthusiast or simply curious about the wonders of nature, white opal is a gemstone that never fails to amaze.
Crystal and Transparent Opals
Opals can vary not only in color but also in their transparency. Some opals are crystal clear and transparent, allowing light to pass through them effortlessly. This transparency is due to the arrangement of silica spheres within the opal's structure. When these spheres are closely packed and of similar size, they create a regular pattern that allows light to pass through, resulting in a transparent opal.
Crystal opal's chemical composition, primarily consisting of hydrated silica, gives rise to its captivating play-of-color and unique optical properties. The presence of impurities and trace elements further enhances its beauty, resulting in a wide range of colors. By understanding the chemical composition of crystal opal, we can appreciate the intricate science behind this enchanting gemstone.
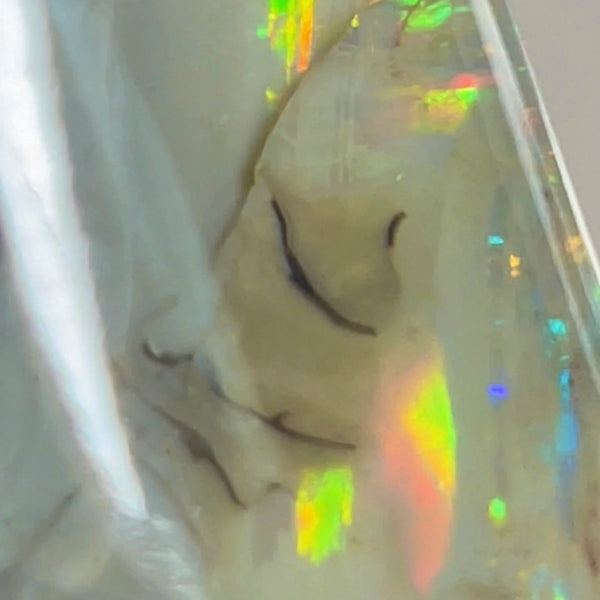
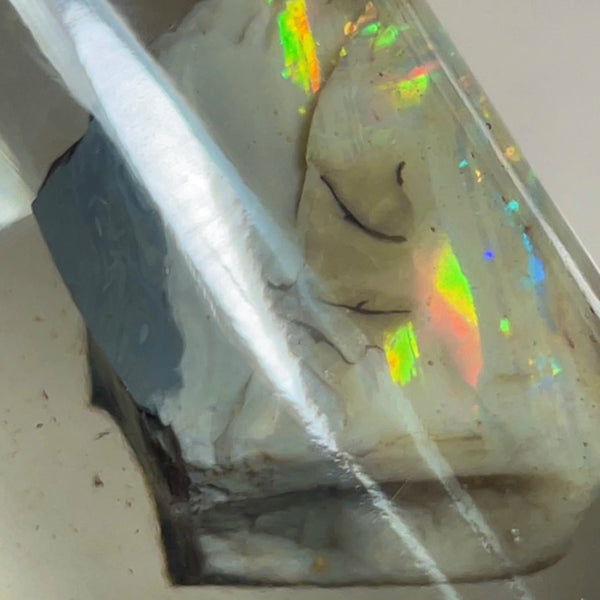
Boulder opal (Ironstone host)
Boulder opal is a unique and captivating gemstone that is highly valued for its stunning play of colors. But have you ever wondered what gives boulder opal its beautiful hues and distinctive patterns? In this blog post, we will explore the chemical composition of boulder opal and delve into the scientific details that make this gemstone so fascinating.
What is Boulder Opal Made Of?
Boulder opal is a type of precious opal that is found in the form of thin veins or patches within ironstone boulders. It is primarily composed of silica, which is the same mineral that makes up quartz and sand. The silica forms tiny spheres or particles that diffract light, resulting in the mesmerizing play of colors that boulder opal is known for.
The Role of Water
One of the key factors that contribute to the formation of boulder opal is the presence of water. Water acts as a catalyst in the process of opalization, which is the transformation of silica into opal. Over millions of years, water carrying dissolved silica seeps into cracks and crevices in the ironstone boulders. As the water evaporates, it leaves behind deposits of silica that gradually solidify into opal.
The Influence of Ironstone
Ironstone, the host rock of boulder opal, plays a crucial role in determining the gemstone's appearance. The iron minerals present in the rock can impart various colors to the opal, ranging from red and orange to blue and green. These vibrant hues are created when light interacts with the iron oxide minerals and the silica spheres within the opal.
Trace Elements and Impurities
In addition to silica and iron, boulder opal can contain trace elements and impurities that contribute to its unique characteristics. These elements can include copper, manganese, and nickel, which can give the opal additional colors and patterns. The presence of these trace elements can also affect the opal's durability and hardness.
The Importance of Composition
Understanding the chemical composition of boulder opal is not only fascinating from a scientific standpoint but also crucial for gemologists and lapidaries. By analyzing the composition of boulder opal, experts can determine its authenticity, origin, and potential treatments. This knowledge allows them to accurately assess the value and quality of the gemstone.
In conclusion, the chemical composition of boulder opal is primarily silica, with the presence of water, ironstone, and trace elements playing significant roles in its formation and appearance. The intricate interplay of these elements gives boulder opal its mesmerizing colors and patterns, making it a truly unique and captivating gemstone.